
Do you know the word "adjuvant"? Most people may be familiar with the word and concept of "vaccines" but not familiar with those of "adjuvants".
The word adjuvant has its origin from the Latin "adjuvare", meaning "to help". It is a general term for substances (factors) which are coadministered with a vaccine with the aim of increasing the effect (immunogenicity) of the vaccine. The research and development of adjuvants has a history of more than 80 years, and they are not a very new medicine. Nevertheless, research into why adjuvants were effective was not much conducted until recently. It was thought that adjuvants merely maintain the stability of vaccine antigens in the body and release them slowly (effect of sustained-release). The actual mechanism was not immunologically understood for a long time, and there was even a sarcastic remark "Immunologist's dirty little secret". However, research in immunology and microbiology for the past ten odd years, especially the research on innate immunity and dendritic cells (awarded 2011 Nobel Prize in Physiology or Medicine) triggered the discovery of a series of findings about adjuvants. Consequently, it allowed the development of adjuvants through an innovative scientific approach, and there is fierce competition worldwide for the development of next-generation adjuvants.
On the other hand, however, adjuvants range widely in terms of origin and mode of action, and they may be the cause or underlying cause of vaccine toxicity, especially immunotoxicity. Nevertheless, efficacy and safety indicators of adjuvants are said to be still underdeveloped compared to the development of other drugs. Furthermore, considering the current situation where adjuvants are added to vaccines being imported from the US and Europe, and vaccine industry and business are being developed explosively in South Korea, China, India, Singapore, etc., further innovation with cooperation and support from industry, academia and government in Japan and overseas, with regulatory bodies involved, is needed to develop adjuvants originating from Japan.
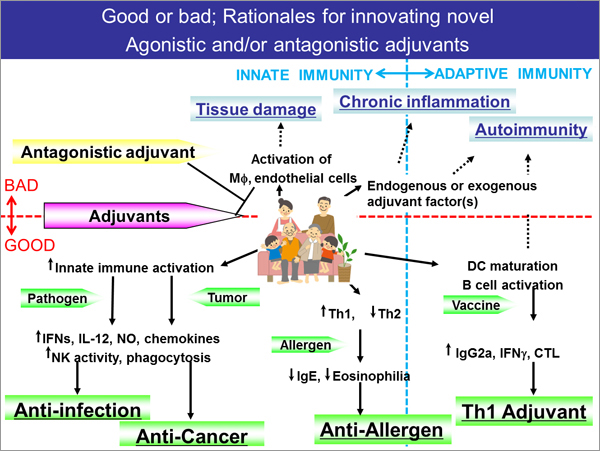
What is needed to make innovation happen in such situation? It is no doubt that advanced technology and scientific knowledge should be utilized for innovation, while the most important task in the development of next-generation adjuvants is probably to establish indicators for "safety". Indicators for efficacy are important; however, in the case of drugs that exhibit strong biological activity, such as adjuvants, the demonstration of high safety profile based on scientific grounds is a key factor to provide the public with good vaccines, also from a viewpoint of "the farthest way about is the nearest way home". This is why new evaluation methods of adjuvants, i.e. the establishment of indicators (biomarkers), is desperately needed throughout the world.
Given the above, the National Institute of Biomedical Innovation (NIBIO) took the initiative in the establishment of Research Group on Next-Generation Adjuvant, founded in fall 2010, by calling on researchers in universities and national research institutes, etc., who are involved in the research and development of adjuvants, as well as pharmaceutical companies involved in adjuvant development, and persons in regulatory organizations in the field of adjuvant development. As of today (March 2012), its meetings were held about 4 times, and a technical book about trends in adjuvant research and development and its future direction was issued (August 2011, CMC Publishing Co., Ltd., Reference 1). In addition to lecturing activities in academic societies and public symposiums, the Research Group is putting a lot of effort into outreach activities, such as introducing adjuvant research at NIBIO's opening to the public, giving lectures to junior high and high school students on demand, handling of inquiries about adverse reactions to vaccines and adjuvants, etc.

In April 2012, under the support of Health and Labour Sciences Research Grants, Adjuvant Database Project was launched on the initiative of the Research Group and NIBIO, in cooperation with universities throughout Japan, national research institutes and testing organizations. This project has just started, but it will establish a database that comprehensively analyzes biological responses to various adjuvants at the human cellular and in vivo level. Preparation for the establishment is currently ongoing.
The detailed contents and roles of this Adjuvant Database Project are given as follows:
- Based on the world-class level Toxicogenomics Database (TG-GATE) established by NIBIO, while following its information and technology, the Project aims for a more innovative database.
- NIBIO's adjuvant development team works with NIBIO's bioinformatics team and collaborative research groups to proceed with the hereinafter described research. That is to say, it aims to analyze biological reaction to adjuvants by gene expression profiling, the latest immunological analysis, and identification and expression profiling of the base sequencing of microRNA in serum from subjects in actual clinical studies and research, and make a database of these. To be specific, the research experiment will use human immune cells and model animals such as mice to conduct genetic analysis of initial reaction to adjuvants and vaccines using DNA array (gene expression profiling) and microRNA array.
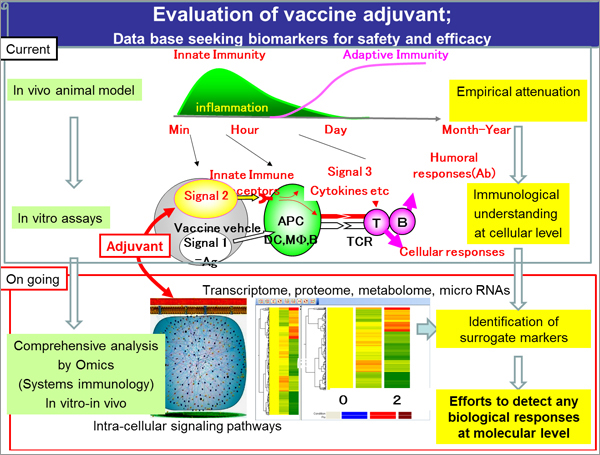
- The participating organizations of this project are the world's leading organizations, including immune-related knockout mice resource (Osaka University, Hyogo College of Medicine, Hokkaido University, Institute of Medical Science, and University of Tokyo) and Tsukuba Primate Research Center (NIBIO), which develop evaluation methods of vaccines and adjuvants, and National Institute of Infectious Diseases, which conducts national testing.
- In addition, similar analysis is to be conducted using samples (such as serum) from actual clinical studies of adjuvanted vaccines in humans. By analyzing target cells for adjuvants and improving imaging technology, it aims to apply spatiotemporal changes after administration of adjuvants (vaccines) to safety and efficacy evaluation methods.
- Information obtained will be stored in the database by using bioinformatics and disclosed to the public as they become available. In addition, most recent algorithm will be used to discover safety and efficacy biomarkers. Ultimately, it aims to develop a system to predict adjuvant safety and develop new methods to evaluate adjuvant safety.
The ultimate goal is to utilize these findings for the prompt improvement of efficacy and safety in collaboration research with companies, and contribute to judgments of the vaccines regulatory agencies and the immunization administration, i.e. the development of safety evaluation methods and the preparation of guidelines. The goal shall always play a part in securing safety in vaccine and adjuvant recipients as well as a sense of security. Furthermore, Research Group on Next-Generation Adjuvant, a joint research among industry, academia and government, has a duty to take a leadership role in disclosing findings and knowledge to the media and the consuming public in cooperation with Japanese Society for Vaccinology, Japanese Society for Immunology, and Japan Pediatric Society.





